News
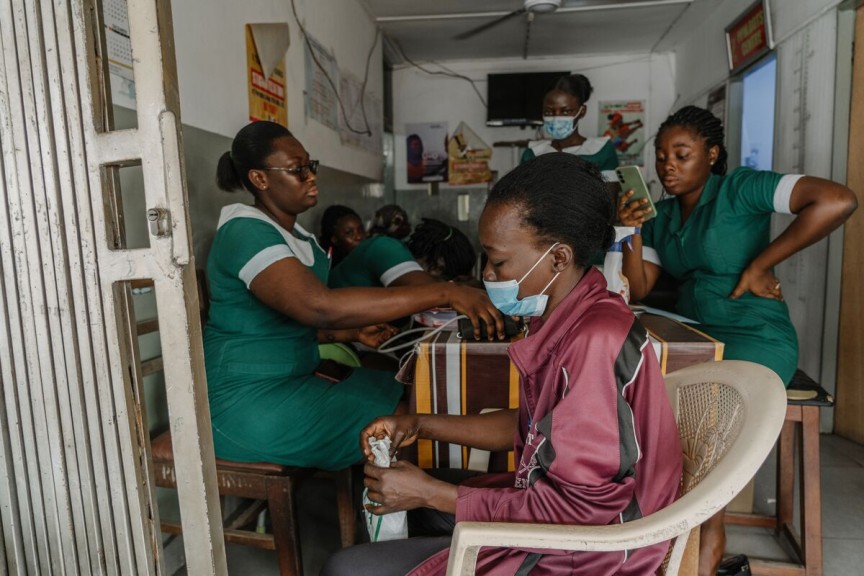
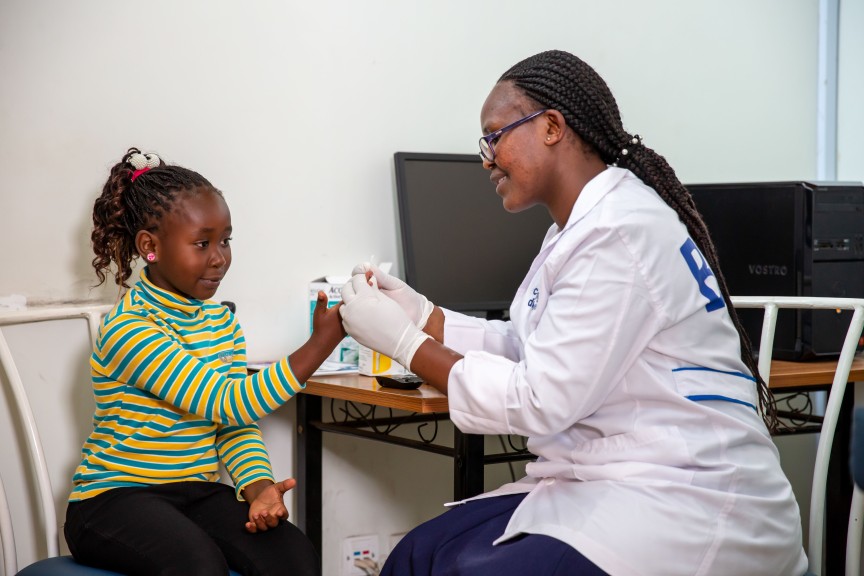
Access to medicine transforms lives. Join us on a mission to reach 8 billion.
Featured insights
Discover the latest at the FoundationExplore per sector
Resource centre
Explore our research reports and publicationsExplore our cross-sector programmes
Events and engagements
SHARING OUR INSIGHTS TO HELP DRIVE CHANGE
Event
Suzi van Es joins EBRD Symposium to discuss public and private cooperation on curbing AMR globally
18 April 2024
Event
Claudia Martínez joins panel at 4th Symposium on Diabetes in Humanitarian Crises
09 May 2024
Event
Amsterdam Session on securing worldwide access to diabetes care by 2030
09 April 2024